 |
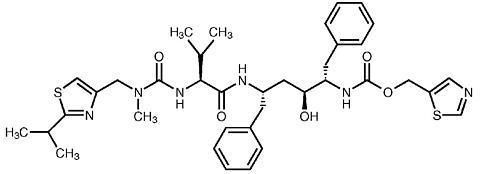
KALETRA (lopinavir/ritonavir) is a co-formulation of lopinavir and ritonavir. Lopinavir is an inhibitor of the HIV protease. As co-formulated in KALETRA, ritonavir inhibits the CYP3A-mediated metabolism of lopinavir, thereby providing increased plasma levels of lopinavir.
Lopinavir is chemically designated as [1S-[1R*,(R*), 3R*, 4R*]]-N-[4-[[(2,6-dimethylphenoxy)acetyl]amino]-3 -hydroxy-5- phenyl-1-(phenylmethyl)pentyl]tetrahydro-alpha-(1-methylethyl)-2-oxo-1(2H)-pyrimidineacetamide. Its molecular formula is C 37 H 48 N 4 O 5 , and its molecular weight is 628.80. Lopinavir has the following structural formula:
 |
Ritonavir is chemically designated as 10-Hydroxy-2-methyl-5-(1-methylethyl)-1-[2-(1-methylethyl)-4-thiazolyl]-3, 6-dioxo- 8,11-bis(phenylmethyl)-2,4,7,12-tetraazatridecan-13-oic acid, 5-thiazolymethyl ester, [5S-(5R*,8R*,10R*,11R*)]. Its molecular formula is C 37 H 48 N 6 O 5 S 2 , and its molecular weight is 720.95. Ritonavir has the following structural formula:
 |
Lopinavir is a white to light tan powder. It is freely soluble in methanol and ethanol, soluble in isopropanol and practically insoluble in water.
KALETRA capsules are available for oral administration in a strength of 133.3 mg lopinavir and 33.3 mg ritonavir with the following inactive ingredients: FD&C Yellow No. 6, gelatin, glycerin, oleic acid, polyoxyl 35 castor oil, propylene glycol, sorbitol special, titanium dioxide, and water.
KALETRA oral solution is available for oral administration as 80 mg lopinavir and 20 mg ritonavir per milliliter with the following inactive ingredients: Acesulfame potassium, alcohol, artificial cotton candy flavor, citric acid, glycerin, high fructose corn syrup, Magnasweet-110 flavor, menthol, natural & artificial vanilla flavor, peppermint oil, polyoxyl 40 hydrogenated castor oil, povidone, propylene glycol, saccharin sodium, sodium chloride, sodium citrate, and water.
KALETRA oral solution contains 42.4% alcohol (v/v).
Mechanism of action: Lopinavir, an inhibitor of the HIV protease, prevents cleavage of the Gag-Pol polyprotein, resulting in the production of immature, non-infectious viral particles.
Antiviral activity in vitro: The in vitro antiviral activity of lopinavir against laboratory HIV strains and clinical HIV isolates was evaluated in acutely infected lymphoblastic cell lines and peripheral blood lymphocytes, respectively. In the absence of human serum, the mean 50% effective concentration (EC 50 ) of lopinavir against five different HIV-1 laboratory strains ranged from 10-27 nM (0.006-0.017 µg/mL, 1 µg/mL = 1.6 µM) and ranged from 4-11 nM (0.003-0.007 µg/mL) against several HIV-1 clinical isolates (n=6). In the presence of 50% human serum, the mean EC 50 of lopinavir against these five laboratory strains ranged from 65-289 nM (0.04-0.18 µg/mL), representing a 7- to 11-fold attenuation. Combination drug activity studies with lopinavir and other protease inhibitors or reverse transcriptase inhibitors have not been completed.
Resistance HIV-1 isolates with reduced susceptibility to lopinavir have been selected in vitro. The presence of ritonavir does not appear to influence the selection of lopinavir-resistant viruses in vitro.
The selection of resistance to KALETRA in antiretroviral treatment naive patients has not yet been characterized. In Phase II studies of 227 antiretroviral treatment naive and protease inhibitor experienced patients, isolates from 4 of 23 patients with quantifiable (>400 copies/mL) viral RNA following treatment with KALETRA for 12 to 100 weeks displayed significantly reduced susceptibility to lopinavir compared to the corresponding baseline viral isolates. Three of these patients had previously received treatment with a single protease inhibitor (nelfinavir, indinavir, or saquinavir) and one patient had received treatment with multiple protease inhibitors (indinavir, saquinavir and ritonavir). All four of these patients had at least 4 mutations associated with protease inhibitor resistance immediately prior to KALETRA therapy. Following viral rebound, isolates from these patients all contained additional mutations, some of which are recognized to be associated with protease inhibitor resistance. However, there are insufficient data at this time to identify lopinavir-associated mutational patterns in isolates from patients on KALETRA therapy. The assessment of these mutational patterns is under study.
Cross-resistance--Preclinical Studies: Varying degrees of cross-resistance have been observed among protease inhibitors. Little information is available on the cross-resistance of viruses that developed decreased susceptibility to lopinavir during KALETRA therapy.
The in vitro activity of lopinavir against clinical isolates from patients previously treated with a single protease inhibitor was determined. Isolates that displayed >4-fold reduced susceptibility to nelfinavir (n=13) and saquinavir (n=4), displayed <4-fold reduced susceptibility to lopinavir. Isolates with >4-fold reduced susceptibility to indinavir (n=16) and ritonavir (n=3) displayed a mean of 5.7- and 8.3-fold reduced susceptibility to lopinavir, respectively. Isolates from patients previously treated with two or more protease inhibitors showed greater reductions in susceptibility to lopinavir, as described in the following paragraph.
Clinical Studies--Antiviral activity of KALETRA in patients with previous protease inhibitor therapy: The clinical relevance of reduced in vitro susceptibility to lopinavir has been examined by assessing the virologic response to KALETRA therapy, with respect to baseline viral genotype and phenotype, in 56 NNRTI-naive patients with HIV RNA >1000 copies/mL despite previous therapy with at least two protease inhibitors selected from nelfinavir, indinavir, saquinavir and ritonavir (Study 957). The EC 50 of lopinavir against the 56 baseline viral isolates ranged from 0.5- to 96-fold higher than the EC 50 against wild type HIV. Fifty-five percent of these baseline isolates displayed a >4-fold reduced susceptibility to lopinavir with a mean reduction in lopinavir susceptibility of 27.9-fold.
After 24 weeks of treatment with KALETRA, efavirenz and nucleoside reverse transcriptase inhibitors, plasma HIV RNA </=400 copies/mL was observed in 93% (27/29) and 65% (15/23) of patients with <10-fold and >/=10-fold reduced susceptibility to lopinavir at baseline, respectively.
In addition, virologic response was observed in 96% (24/25) of patients whose baseline viral isolates contained up to 5 mutations recognized to be associated with protease inhibitor resistance. Fourteen of those 25 isolates contained mutations at position 82, 84 and/or 90. Virologic response was observed in 67% (18/27) of patients whose baseline viral isolates contained 6 or more mutations, including those at positions 82, 84 and/or 90 plus multiple other mutations. There are insufficient data at this time to identify lopinavir-associated mutational patterns in isolates from patients on KALETRA therapy. Further studies are needed to assess the association between specific mutational patterns and virologic response rates.
The pharmacokinetic properties of lopinavir co-administered with ritonavir have been evaluated in healthy adult volunteers and in HIV-infected patients; no substantial differences were observed between the two groups. Lopinavir is essentially completely metabolized by CYP3A. Ritonavir inhibits the metabolism of lopinavir, thereby increasing the plasma levels of lopinavir. Across studies, administration of KALETRA 400/100 mg BID yields mean steady-state lopinavir plasma concentrations 15- to 20-fold higher than those of ritonavir in HIV-infected patients. The plasma levels of ritonavir are less than 7% of those obtained after the ritonavir dose of 600 mg BID. The in vitro antiviral EC 50 of lopinavir is approximately 10-fold lower than that of ritonavir. Therefore, the antiviral activity of KALETRA is due to lopinavir.
Figure 1 displays the mean steady-state plasma concentrations of lopinavir and ritonavir after KALETRA 400/100 mg BID for 3-4 weeks from a pharmacokinetic study in HIV-infected adult subjects (n=21).
 |
Absorption In a pharmacokinetic study in HIV-positive subjects (n=21) without meal restrictions, multiple dosing with 400/100 mg KALETRA BID for 3 to 4 weeks produced a mean ± SD lopinavir peak plasma concentration (C max ) of 9.6 ± 4.4 µg/mL, occurring approximately 4 hours after administration. The mean steady-state trough concentration prior to the morning dose was 5.5 ± 4.0 µg/mL. Lopinavir AUC over a 12 hour dosing interval averaged 82.8 ± 44.5 µg•h/mL. The absolute bioavailability of lopinavir co-formulated with ritonavir in humans has not been established. Under nonfasting conditions (500 kcal, 25% from fat), lopinavir concentrations were similar following administration of KALETRA co-formulated capsules and liquid. When administered under fasting conditions, both the mean AUC and C max of lopinavir were 22% lower for the KALETRA liquid relative to the capsule formulation.
Effects of Food on Oral Absorption: Administration of a single 400/100 mg dose of KALETRA capsules with a moderate fat meal (500-682 kcal, 23 to 25% calories from fat) was associated with a mean increase of 48 and 23% in lopinavir AUC and C max , respectively, relative to fasting. For KALETRA oral solution, the corresponding increases in lopinavir AUC and C max were 80 and 54%, respectively. Relative to fasting, administration of KALETRA with a high fat meal (872 kcal, 56% from fat) increased lopinavir AUC and C max by 97 and 43%, respectively, for capsules, and 130 and 56%, respectively, for oral solution. To enhance bioavailability and minimize pharmacokinetic variability KALETRA should be taken with food.
Distribution: At steady state, lopinavir is approximately 98-99% bound to plasma proteins. Lopinavir binds to both alpha-1-acid glycoproteins (AAG) and albumin; however, it has a higher affinity for AAG. At steady state, lopinavir protein binding remains constant over the range of observed concentrations after 400/100 mg KALETRA BID, and is similar between healthy volunteers and HIV-positive patients.
Metabolism In vitro experiments with human hepatic microsomes indicate that lopinavir primarily undergoes oxidative metabolism. Lopinavir is extensively metabolized by the hepatic cytochrome P450 system, almost exclusively by the CYP3A isozyme. Ritonavir is a potent CYP3A inhibitor which inhibits the metabolism of lopinavir, and therefore increases plasma levels of lopinavir. A 14 C-lopinavir study in humans showed that 89% of the plasma radioactivity after a single 400/100 mg KALETRA dose was due to parent drug. At least 13 lopinavir oxidative metabolites have been identified in man. Ritonavir has been shown to induce metabolic enzymes, resulting in the induction of its own metabolism. Pre-dose lopinavir concentrations decline with time during multiple dosing, stabilizing after approximately 10 to 16 days.
Elimination Following a 400/100 mg 14 C-lopinavir/ritonavir dose, approximately 10.4 ± 2.3% and 82.6 ± 2.5% of an administered dose of 14 C-lopinavir can be accounted for in urine and feces, respectively, after 8 days. Unchanged lopinavir accounted for approximately 2.2 and 19.8% of the administered dose in urine and feces, respectively. After multiple dosing, less than 3% of the lopinavir dose is excreted unchanged in the urine. The half-life of lopinavir over a 12 hour dosing interval averaged 5-6 hours, and the apparent oral clearance (CL/F) of lopinavir is 6 to 7 L/h.
Gender, Race and Age : Lopinavir pharmacokinetics have not been studied in elderly patients. No gender related pharmacokinetic differences have been observed in adult patients. No clinically important pharmacokinetic differences due to race have been identified.
Pediatric Patients : The pharmacokinetics of KALETRA 300/75 mg/m 2 BID and 230/57.5 mg/m 2 BID have been studied in a total of 53 pediatric patients, ranging in age from 6 months to 12 years. The 230/57.5 mg/m 2 BID regimen without nevirapine and the 300/75 mg/m 2 BID regimen with nevirapine provided lopinavir plasma concentrations similar to those obtained in adult patients receiving the400/100 mg BID regimen (without nevirapine).
The lopinavir mean steady-state AUC, C max , and C min were 72.6 ± 31.1 µg•h/mL, 8.2 ± 2.9 and 3.4 ± 2.1 µg/mL, respectively after KALETRA 230/57.5 mg/m 2 BID without nervirapine (n=12), and were 85.8 ± 36.9 µg•h/mL, 10.0 ± 3.3 and 3.6 ± 3.5 µg/mL, respectively after 300/75 mg/m 2 BID with nevirapine (n=12). The nevirapine regimen was 7 mg/kg BID (3 months to 8 years) or 4 mg/kg BID (>8 years).
Renal Insufficiency : Lopinavir pharmacokinetics have not been studied in patients with renal insufficiency; however, since the renal clearance of lopinavir is negligible, a decrease in total body clearance is not expected in patients with renal insufficiency.
Hepatic Impairment : Lopinavir is principally metabolized and eliminated by the liver. Although KALETRA has not been studied in patients with hepatic impairment, lopinavir concentrations may be increased in these patients (see PRECAUTIONS ).
Drug-Drug Interactions : See also CONTRAINDICATIONS , WARNINGS and PRECAUTIONS : Drug Interactions .
KALETRA is an inhibitor of the P450 isoform CYP3A in vitro . Co-administration of KALETRA and drugs primarily metabolized by CYP3A may result in increased plasma concentrations of the other drug, which could increase or prolong its therapeutic and adverse effects (see CONTRAINDICATIONS ).
KALETRA inhibits CYP2D6 in vitro , but to a lesser extent than CYP3A. Clinically significant drug interactions with drugs metabolized by CYP2D6 are possible with KALETRA at the recommended dose, but the magnitude is not known. KALETRA does not inhibit CYP2C9, CYP2C19, CYP2E1, CYP2B6 or CYP1A2 at clinically relevant concentrations.
KALETRA has been shown in vivo to induce its own metabolism and to increase the biotransformation of some drugs metabolized by cytochrome P450 enzyme and by glucuronidation.
KALETRA is metabolized by CYP3A. Drugs that induce CYP3A activity would be expected to increase the clearance of lopinavir, resulting in lowered plasma concentrations of lopinavir. Although not noted with concurrent ketoconazole, co-administration of KALETRA and other drugs that inhibit CYP3A may increase lopinavir plasma concentrations.
Drug interaction studies were performed with KALETRA and other drugs likely to be co-administered and some drugs commonly used as probes for pharmacokinetic interactions. The effects of co-administration of KALETRA of the AUC, C max and C min are summarized in Table 1 (effect of other drugs on lopinavir) and Table 2 (effect of KALETRA on other drugs). The effects of other drugs on ritonavir are not shown since they generally correlate with those observed with lopinavir (if lopinavir concentrations are decreased, ritonavir concentrations are decreased) unless otherwise indicated in the table footnotes. For information regarding clinical recommendations, see Table 6 in PRECAUTIONS .
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Effect of KALETRA on other Protease Inhibitors (PIs): The pharmacokinetics of single-dose indinavir and saquinavir, and multiple-dose amprenavir obtained in healthy subjects after at least 10 days of KALETRA 400/100 mg BID were compared to historical data in HIV-infected subjects (refer to Table 2 for information on study design and doses). Because of the limitations in the study design and the use of comparisons between healthy and HIV infected subjects, it is not possible to recommend definitive dosing recommendations. However, based on these comparisons, amprenavir 750 mg BID and indinavir 600 mg BID, when co-administered with KALETRA 400/100 mg BID, may produce a similar AUC, lower C max , and higher C min compared to their respective established clinical dosing regimens. Saquinavir 800 mg BID, when co-administered with KALETRA 400/100 mg BID, may produce a similar AUC and higher C min to its respective established clinical dosing regimen (no comparative information regarding C max ). The clinical significance of the lower C max and higher C min is unknown. Appropriate doses of amprenavir, indinavir and saquinavir in combination with KALETRA with respect to safety and efficacy have not been established (see PRECAUTIONS --Table 6).
KALETRA is indicated in combination with other antiretroviral agents for the treatment of HIV-infection. This indication is based on analyses of plasma HIV RNA levels and CD4 cell counts in a controlled study of KALETRA of 24 weeks duration and in smaller uncontrolled dose-ranging studies of KALETRA of 72 weeks duration. At present, there are no results from controlled trials evaluating the effect of KALETRA on clinical progression of HIV.
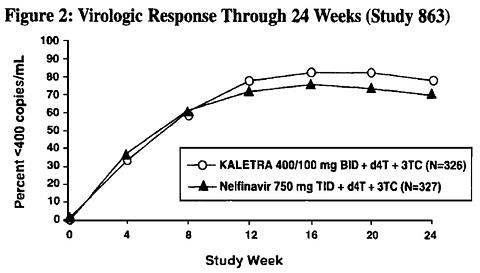
Study 863: KALETRA BID + stavudine + lamivudine compared to nelfainavir TID + stavudine + lamivudine
Study 863 is an ongoing, randomized, double-blind, multicenter trial comparing treatment with KALETRA(400/100 mg BID) plus stavudine and lamivudine versus nelfinavir (750 mg TID) plus stavudine and lamivudine in 653 antiretroviral treatment naive patients. Patients had a mean age of 38 years (range: 19 to 84), 57% were Caucasian, and 80% were male. Mean baseline CD4 cell count was 259 cells/mm 3 (range: 2 to 949 cells/mm 3 ) and mean baseline plasma HIV-1 RNA was 4.9 log 10 copies/mL (range: 2.6 to 6.8 log 10 copies/mL).
The percent of patients with HIV RNA <400 copies/mL and outcomes of patients through 24 weeks are summarized in Figure 2 and Table 3, respectively.
 |
|
|||||||||||||||||||||||||||
In the KALETRA arm, the proportion <400 copies/mL for patients with baseline HIV RNA >100,000 copies/mL (78%) was similar to that for patients with baseline HIV RNA <100,000 copies/mL (81%).
Through 24 weeks of therapy, the proportion of patients with HIV RNA <50 copies/mL was 65% in the KALETRA arm and 60% in the nelfinavir arm.
Through 24 weeks of therapy, the mean increase from baseline in CD4 cell count was 154 cells/mm 3 for the KALETRA arm and 150 cells/mm 3 for the nelfinavir arm.
Four patients in the KALETRA arm and 6 patients in the nelfinavir arm experienced a new CDC Class C event following at least one week of treatment, including 3 patients in each arm who achieved HIV RNA <400 copies/mL at 24 weeks.
Study 720: KALETRA BID + stavudine + lamivudine
Study 720 is an ongoing, randomized, blinded, multicenter trial evaluating treatment with KALETRA at three dose levels (Group I: 200/100 mg BID and 400/100 mg BID; Group II: 400/100 mg BID and 400/200 mg BID) plus lamivudine (150 mg BID) and stavudine (40 mg BID) in 100 patients. All patients were converted to open-label KALETRA at the 400/100 mg BID dose between weeks 48 and 72 of the study. Patients had a mean age of 35 years (range: 21 to 59), 70% were Caucasian, and 96% were male. Mean baseline CD4 cell count was 338 cells/mm 3 (range: 3 to 918 cells/mm 3 ) and mean baseline plasma HIV-1 RNA was 4.9 log 10 copies/mL (range: 3.3 to 6.3 log 10 copies/mL).
Through 72 weeks of treatment, the proportion of patients with HIV RNA <400 (<50) copies/mL was 80% (78%) and the mean increase from baseline in CD4 cell count was 256 cells/mm 3 for the 51 patients originally randomized to the 400/100 mg dose of KALETRA. At 72 weeks, 13 patients (13%) had discontinued the study for any reason. Four discontinuations (4%) were secondary to adverse events or laboratory abnormalities, and one of these discontinuations (1%) was attributed to a KALETRA adverse event.
Study 765: KALETRA BID + nevirapine + NRTIs
Study 765 is an ongoing, randomized, blinded, multicenter trial evaluating treatment with KALETRA at two dose levels (400/100 mg BID and 400/200 mg BID) plus nevirapine (200 mg BID) and two NRTIs in 70 single protease inhibitor experienced, non-nucleoside reverse transcriptase inhibitor (NNRTI) naive patients. Patients had a mean age of 40 years (range 22-66), were 73% were Caucasian, and were 90% male. Mean baseline CD4 cell count was 372 cells/mm 3 (range 72 to 807 cells/µL) and mean baseline plasma HIV-1 RNA was 4.0 log 10 copies/mL (range 2.9 to 5.8 log 10 copies/mL).
Through 72 weeks of treatment, the proportion of patients with HIV RNA <400 (<50) copies/mL was 75% (58%) and the mean increase from baseline in CD4 cell count was 174 cells/mm 3 for the 36 patients receiving the 400/100 mg dose of KALETRA. At 72 weeks, 13 patients (19%) had discontinued the study for any reason. Six discontinuations (9%) were secondary to adverse events or laboratory abnormalities, and three of these discontinuations (4%) was attributed to KALETRA adverse event.
KALETRA is contraindicated in patients with known hypersensitivty to any of its ingredients, including ritonavir.
Co-administration of KALETRA is contraindicated with drugs that are highly dependent on CYP3A or CYP2D6 for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening events. These drugs are listed in Table 4.
|
ALERT: Find out about medicines that should NOT be taken with KALETRA. This statement is included on the product' bottle label.
KALETRA is an inhibitor of the P450 isoform CYP3A. Co-administration of KALETRA and drugs primarily metabolized by CYP3A or CYP2D6 may result in increased plasma concentrations of the other drug that could increase or prolong its therapeutic and adverse effect (see Pharmacokinetics: Drug-Drug Interactions, CONTRAINDICATIONS --Table 4: Drugs That Are Contraindicated With KALETRA, PRECAUTIONS --Table 5: Drugs That Should Not Be Co-administered with KALETRA and Table 6: Established and Other Potentially Significant Drug Interactions ).
Particular caution should be used when prescribing sildenafil in patients receiving KALETRA. Co-administration of KALETRA with sildenafil is expected to substantially increase sildenafil concentrations and may result in an increase in sildenafil-associated adverse events including hypotension, syncope, visual changes and prolonged erection (see PRECAUTIONS : Drug Interactions and the complete prescribing information for sildenafil).
Concomitant use of KALETRA with lovastatin or simvastatin is not recommended. Caution should be exercised if HIV protease inhibitors, including KALETRA, are used concurrently with other HMG-CoA reductase inhibitors that are also metabolized by the CYP3A4 pathway (e.g., atorvastatin or cerivastatin). The risk of myopathy, including rhabdomyolysis may be increased when HIV protease inhibitors, including KALETRA, are used in combination with these drugs.
Concomitant use of KALETRA and St. John's wort (hypericum perforatum), or products containing St. John's wort, is not recommended. Co-administration of protease inhibitors, including KALETRA, with St. John's wort is expected to substantially decrease protease inhibitor concentrations and may result in sub-optimal levels of lopinavir and lead to loss of virologic response and possible resistance to lopinavir or to the class of protease inhibitors.
Pancreatitis has been observed in patients receivingKALETRA therapy, including those who developed marked triglyceride elevations. In some cases, fatalities have been observed. Although a causal relationship to KALETRA has not been established, marked triglyceride elevations is a risk factor for development of pancreatitis (see PRECAUTIONS -- Lipid Elevations ). Patients with advanced HIV disease may be at increased risk of elevated triglycerides and pancreatitis, and patients with a history of pancreatitis may be at increased risk of recurrence during KALETRA therapy.
Pancreatitis should be considered if clinical symptoms (nausea, vomiting, abdominal pain) or abnormalities in laboratory values (such as increased serum lipase or amylase values) suggestive of pancreatitis should occur. Patients who exhibit these signs or symptoms should be evaluated and KALETRA and/or other antiretroviral therapy should be suspended as clinically appropriate.
New onset diabetes mellitus, exacerbation of pre-existing diabetes mellitus, and hyperglycemia have been reported during postmarketing surveillance in HIV-infected patients receiving protease inhibitor therapy. Some patients required either initiation or dose adjustments of insulin or oral hypoglycemic agents for treatment of these events. In some cases, diabetic ketoacidosis has occurred. In those patients who discontinued protease inhibitor therapy, hyperglycemia persisted in some cases. Because these events have been reported voluntarily during clinical practice, estimates of frequency cannot be made and a causal relationship between protease inhibitor therapy and these events has not been established.
KALETRA is principally metabolized by the liver; therefore, caution should be exercised when administering this drug to patients with hepatic impairment, because lopinavir concentrations may be increased. Patients with underlying hepatitis B or C or marked elevations in transaminases prior to treatment may be at increased risk for developing further transaminase elevations.
Various degrees of cross-resistance among protease inhibitors have been observed. The effect of KALETRA therapy on the efficacy of subsequently administered protease inhibitors is under investigation (see MICROBIOLOGY ).
There have been reports of increased bleeding, including spontaneous skin hematomas and hemarthrosis, in patients with hemophilia type A and B treated with protease inhibitors. In some patients additional factor VIII was given. In more than half of the reported cases, treatment with protease inhibitors was continued or reintroduced. A causal relationship between protease inhibitor therapy and these events has not been established.
Redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, breast enlargement, and "cushingoid appearance" have been observed in patients receiving antiretroviral therapy. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established.
Treatment with KALETRA has resulted in large increases in the concentration of total cholesterol and triglycerides (see ADVERSE REACTIONS --Table 8). Triglyceride and cholesterol testing should be performed prior to initiating KALETRA therapy and at periodic intervals during therapy. Lipid disorders should be managed as clinically appropriate. See PRECAUTIONS Table 6: Established and Other Potentially Significant Drug Interactions for additional information on potential drug interactions with KALETRA and HMG-CoA reductase inhibitors.
A statement to patients and health care providers is included on the product' bottle label: ALERT: Find out about medicines that should NOT be taken with KALETRA. A Patient Package Insert (PPI) for KALETRA is available for patient information.
Patients should be told that sustained decreases in plasma HIV RNA have been associated with a reduced risk of progression to AIDS and death. Patients should remain under the care of a physician while using KALETRA. Patients should be advised to take KALETRA and other concomitant antiretroviral therapy every day as prescribed. KALETRA must always be used in combination with other antiretroviral drugs. Patients should not alter the dose or discontinue therapy without consulting with their doctor. If a dose of KALETRA is missed patients should take the dose as soon as possible and then return to their normal schedule. However, if a dose is skipped the patient should not double the next dose.
Patients should be informed that KALETRA is not a cure for HIV infection and that they may continue to develop opportunistic infections and other complications associated with HIV disease. The long-term effects of KALETRA are unknown at this time. Patients should be told that there are currently no data demonstrating that therapy with KALETRA can reduce the risk of transmitting HIV to others through sexual contact.
KALETRA may interact with some drugs; therefore, patients should be advised to report to their doctor the use of any other prescription, non-prescription medication or herbal products, particularly St. John's wort.
Patients taking didanosine should take didanosine one hour before or two hours after KALETRA.
Patients receiving sildenafil should be advised that they may be at an increased risk of sildenafil-associated adverse events including hypotension, visual changes, and sustained erection, and should promptly report any symptoms to their doctor.
Patients receiving estrogen-based hormonal contraceptives should be instructed that additional or alternate contraceptive measures should be used during therapy with KALETRA.
KALETRA should be taken with food to enhance absorption.
Patients should be informed that redistribution or accumulation of body fat may occur in patients receiving antiretroviral therapy including protease inhibitors and that the cause and long-term health effects of these conditions are not known at this time.
KALETRA is an inhibitor of CYP3A (cytochrome P450 3A) both in vitro and in vivo . Co-administration of KALETRA and drugs primarily metabolized by CYP3A (e.g., dihydropyridine calcium channel blockers, HMG-CoA reductase inhibitors, immunosuppressants and sildenafil) may result in increased plasma concentrations of the other drugs that could increase or prolong their therapeutic and adverse effects (see Table 6: Established and Other Potentially Significant Drug Interactions ). Agents that are extensively metabolized by CYP3A and have high first pass metabolism appear to be the most susceptible to large increases in AUC (>3-fold) when co-administered with KALETRA.
KALETRA inhibits CYP2D6 in vitro, but to a lesser extent than CYP3A. Clinically significant drug interactions with drugs metabolized by CYP2D6 are possible with KALETRA at the recommended dose, but the magnitude is not known. KALETRA does not inhibit CYP2C9, CYP2C19, CYP2E1, CYP2B6 or CYP1A2 at clinically relevant concentrations.
KALETRA has been shown in vivo to induce its own metabolism and to increase the biotransformation of some drugs metabolized by cytochrome P450 enzymes and by glucuronidation.
KALETRA is metabolized by CYP3A. Co-administration of KALETRA and drugs that induce CYP3A may decrease lopinavir plasma concentrations and reduce its therapeutic effect (see Table 6: Established and Other Potentially Significant Drug Interactions ). Although not noted with concurrent ketoconazole, co-administration of KALETRA and other drugs that inhibit CYP3A may increase lopinavir plasma concentrations.
Drugs that are contraindicated and not recommended for co-administration with KALETRA are included in Table 5: Drugs That Should Not be Co-administered With KALETRA . These recommendations are based on either drug interaction studies or predicted interactions due to the expected magnitude of interaction and potential for serious events or loss of efficacy.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug interaction studies reveal no clinically significant interaction between KALETRA and pravastatin, stavudine or lamivudine.
Based on known metabolic profiles, clinically significant drug interactions are not expected between KALETRA and fluvastatin, dapsone, trimethoprim/sulfamethoxazole, azithromycin, erythromycin, or fluconazole.
Zidovudine and Abacavir: KALETRA induces glucuronidation; therefore, KALETRA has the potential to reduce zidovudine and abacavir plasma concentrations. The clinical significance of this potential interaction is unknown.
Long-term carcinogenicity studies of KALETRA in animal systems have not been completed.
Carcinogenicity studies in mice and rats have been carried out on ritonavir. In male mice, at levels of 50, 100 or 200 mg/kg/day, there was a dose dependent increase in the incidence of both adenomas and combined adenomas and carcinomas in the liver. Based on AUC measurements, the exposure at the high dose was approximately 4-fold for males that of the exposure in humans with the recommended therapeutic dose (400/100 mg KALETRA BID). There were no carcinogenic effects seen in females at the dosages tested. The exposure at the high dose was approximately 9-fold for the females that of the exposure in humans. In rats dosed at levels of 7, 15 or 30 mg/kg/day there were no carcinogenic effects. In this study, the exposure at the high dose was approximately 0.7-fold that of the exposure in humans with the 400/100 mg KALETRA BID regimen. Based on the exposures achieved in the animal studies, the significance of the observed effects is not known. However, neither lopinavir nor ritonavir was found to be mutagenic or clastogenic in a battery of in vitro and in vivo assays including the Ames bacterial reverse mutation assay using S. typhimurium and E. coli , the mouse lymphoma assay, the mouse micronucleus test and chromosomal aberration assays in human lymphocytes.
Lopinavir in combination with ritonavir at a 2:1 ratio produced no effects on fertility in male and female rats at levels of 10/5, 30/15 or 100/50 mg/kg/day. Based on AUC measurements, the exposures in rats at the high doses were approximately 0.7-fold for lopinavir and 1.8-fold for ritonavir of the exposures in humans at the recommended therapeutic dose (400/100 mg BID).
Pregnancy Category C: No treatment-related malformations were observed when lopinavir in combination with ritonavir was administered to pregnant rats or rabbits. Embryonic and fetal developmental toxicities (early resorption, decreased fetal viability, decreased fetal body weight, increased incidence of skeletal variations and skeletal ossification delays) occurred in rats at a maternally toxic dosage (100/50 mg/kg/day). Based on AUC measurements, the drug exposures in rats at 100/50 mg/kg/day were approximately 0.7-fold for lopinavir and 1.8-fold for ritonavir for males and females that of the exposures in humans at the recommended therapeutic dose (400/100 mg BID). In a peri- and postnatal study in rats, a developmental toxicity (a decrease in survival in pups between birth and postnatal day 21) occurred at 40/20 mg/kg/day and greater.
No embryonic and fetal developmental toxicities were observed in rabbits at a maternally toxic dosage (80/40 mg/kg/day). Based on AUC measurements, the drug exposures in rabbits at 80/40 mg/kg/day were approximately 0.6-fold for lopinavir and 1.0-fold for ritonavir that of the exposures in humans at the recommended therapeutic dose (400/100 mg BID). There are, however, no adequate and well-controlled studies in pregnant women. KALETRA should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Antiretroviral Pregnancy Registry: To monitor maternal-fetal outcomes of pregnant women exposed to KALETRA, an Antiretroviral Pregnancy Registry has been established. Physicians are encouraged to register patients by calling 1-800-258-4263.
Nursing Mothers: The Centers for Disease Control and Prevention recommend that HIV-infected mothers not breast-feed their infants to avoid risking postnatal transmission of HIV. Studies in rats have demonstrated that lopinavir is secreted in milk. It is not known whether lopinavir is secreted in human milk. Because of both the potential for HIV transmission and the potential for serious adverse reactions in nursing infants, mothers should be instructed not to breast-feed if they are receiving KALETRA.
Clinical studies of KALETRA did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, appropriate caution should be exercised in the administration and monitoring of KALETRA in elderly patients reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
The safety and pharmacokinetic profiles of KALETRA in pediatric patients below the age of 6 months have not been established. In HIV-infected patients age 6 months to 12 years, the adverse event profile seen during a clinical trial was similar to that for adult patients. The evaluation of the antiviral activity of KALETRA in pediatric patients in clinical trials is ongoing.
Study 940 is an ongoing open-label, multicenter trial evaluating the pharmacokinetic profile, tolerability, safety and efficacy of KALETRA oral solution containing lopinavir 80 mg/mL and ritonavir 20 mg/mL in 100 antiretroviral naive (44%) and experienced (56%) pediatric patients. All patients were non-nucleoside reverse transcriptase inhibitor naive. Patients were randomized to either 230 mg lopinavir/57.5 mg ritonavir per m 2 or 300 mg lopinavir/75 mg ritonavir per m 2 . Naive patients also received lamivudine and stavudine. Experienced patients received nevirapine plus up to two nucleoside reverse transcriptase inhibitors.
Safety, efficacy and pharmacokinetic profiles of the two dose regimens were assessed after three weeks of therapy in each patient. After analysis of these data, all patients were continued on the 300 mg lopinavir/75 mg ritonavir per m 2 dose. Patients had a mean age of 5 years (range 6 months to 12 years) with 14% less than 2 years. Mean baseline CD4 cell count was 838 cells/mm 3 and mean baseline plasma HIV-1 RNA was 4.7 log 10 copies/mL.
Through 24 weeks of therapy, the proportion of patients with HIV RNA <400 copies/mL was 82% for antiretroviral naive patients and 66% for antiretroviral experienced patients. The mean increase from baseline in CD4 cell count was 328 cells/mm 3 for antiretroviral naive and 335 cells/mm 3 for antiretroviral experienced patients treated through 24 weeks. At 24 weeks, one patient (1%) had prematurely discontinued the study. This discontinuation was secondary to an HIV-related event in an antiretroviral experienced patient that was not attributed to a KALETRA adverse event.
Dose selection for patients 6 months to 12 years of age was based on the following results. The 230/57.5 mg/m 2 BID regimen without nevirapine and the 300/75 mg/m 2 BID regimen with nevirapine provided lopinavir plasma concentrations similar to those obtained in adult patients receiving the 400/100 mg BID regimen (without nevirapine).
Treatment-Emergent Adverse Events: KALETRA has been studied in 612 patients as combination therapy in Phase I/II and Phase III clinical trials. The most common adverse event associated with KALETRA therapy was diarrhea, which was generally of mild to moderate severity. Rates of discontinuation of randomized therapy due to adverse events were 2.8% in KALETRA and 3.1% in nelfinavir treated patients in Study 863.
Drug related clinical adverse events of moderate or severe intensity in >/= 2% of patients treated with combination therapy including KALETRA for up to 24 weeks (Phase III) and for up to 72 weeks (Phase I/II) are presented in Table 7. For other information regarding observed or potentially serious adverse events, please see WARNINGS and PRECAUTIONS .
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Treatment-emergent adverse events occurring in less than 2% of adult patients receiving KALETRA in all phase II/III clinical trials and considered at least possibly related or of unknown relationship to treatment with KALETRA and of at least moderate intensity are listed below by body system.
Body as a Whole: Back pain, chest pain, chest pain substernal, chills, drug interaction, drug level increased, face edema, fever, flu syndrome, malaise, and viral infection.
Cardiovascular System: Deep vein thrombosis, hypertension, palpitation, thrombophlebitis, and vasculitis.
Digestive System: Anorexia, cholecystitis, constipation, dry mouth, dyspepsia, dysphagia, enterocolitis, eructation, esophagitis, fecal incontinence, flatulence, gastritis, gastroenteritis, gastrointestinal disorder, hemorrhagic colitis, increased appetite, pancreatitis, sialadenitis, stomatitis, and ulcerative stomatitis.
Endocrine System: Cushing's syndrome and hypothyroidism.
Hemic and Lymphatic System: Anemia, leukopenia, and lymphadenopathy.
Metabolic and Nutritional Disorders: Avitaminosis, dehydration, edema, glucose tolerance decreased, lactic acidosis, obesity, peripheral edema, and weight loss.
Musculoskeletal System: Arthralgia, arthrosis and myalgia.
Nervous System: Abnormal dreams, agitation, amnesia, anxiety, ataxia, confusion, depression, dizziness, dyskinesia, emotional lability, encephalopathy, hypertonia, libido decreased, nervousness, neuropathy, paresthesia, peripheral neuritis, somnolence, thinking abnormal, and tremor.
Respiratory System: Bronchitis, dyspnea, lung edema, and sinusitis.
Skin and Appendages: Acne, alopecia, dry skin, exfoliative dermatitis, furunculosis, maculopapular rash, nail disorder, pruritis, skin benign neoplasm, skin discoloration, and sweating.
Special Senses: Abnormal vision, eye disorder, otitis media, taste perversion, and tinnitus.
Urogenital System: Abnormal ejaculation, gynecomastia, hypogonadism male, kidney calculus, and urine abnormality.
Laboratory Abnormalities: The percentages of adult patients treated with combination therapy including KALETRA with Grade 3-4 laboratory abnormalities are presented in Table 8.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Treatment-Emergent Adverse Events: KALETRA has been studied in 100 pediatric patients 6 months to 12 years of age. The adverse event profile seen during a clinical trial was similar to that for adult patients.
Rash (2%) was the only drug-related clinical adverse event of moderate or severe intensity in >/= 2% of pediatric patients treated with combination therapy including KALETRA (300/75 mg/m 2 ) for up to 24 weeks (Study 940). This includes adverse events of at least possible, probable or unknown relationship to study drug.
Laboratory Abnormalities: The percentages of pediatric patients treated with combination therapy including KALETRA with Grade 3-4 laboratory abnormalities are presented in Table 9.
|
||||||||||||||||||||||||||||||||||||||||||
KALETRA oral solution contains 42.4% alcohol (v/v). Accidental ingestion of the product by a young child could result in significant alcohol-related toxicity and could approach the potential lethal dose of alcohol.
Human experience of acute overdosage with KALETRA is limited. Treatment of overdose with KALETRA should consist of general supportive measures including monitoring of vital signs and observation of the clinical status of the patient. There is no specific antidote for overdose with KALETRA. If indicated, elimination of unabsorbed drug should be achieved by emesis or gastric lavage. Administration of activated charcoal may also be used to aid in removal of unabsorbed drug. Since KALETRA is highly protein bound, dialysis is unlikely to be beneficial in significant removal of the drug.
The recommended dosage of KALETRA is 400/100 mg (3 capsules or 5.0 mL) twice daily taken with food.
Concomitant therapy : Efavirenz or nevirapine: A dose increase of KALETRA to 533/133 mg (4 capsules or 6.5 mL) twice daily taken with food should be considered when used in combination with efavirenz or nevirapine in treatment experienced patients where reduced susceptibility to lopinavir is clinically suspected (by treatment history or laboratory evidence) (see CLINICAL PHARMACOLOGY --Drug Interactions and/or PRECAUTIONS --Table 6).
In children 6 months to 12 years of age, the recommended dosage of KALETRA oral solution is 12/3 mg/kg for those 7 to <15 kg and 10/2.5 mg/kg for those 15 to 40 kg (approximately equivalent to 230/57.5 mg/m 2 ) twice daily taken with food, up to a maximum dose of 400/100 mg in children >40 kg (5.0 mL or 3 capsules) twice daily. It is preferred that the prescriber calculate the appropriate milligram dose for each individual child </= 12 years old and determine the corresponding volume of solution or number of capsules. However, as an alternative, the following table contains dosing guidelines for KALETRA oral solution based on body weight. When possible, dose should be administered using a calibrated dosing syringe.
|
|||||||||||||||||||||||||||||||||||||||
Concomitant therapy : Efavirenz or nevirapine: A dose increase of KALETRA oral solution to 13/3.25 mg/kg for those 7 to <15 kg and 11/2.75 mg/kg for those 15 to 45 kg (approximately equivalent to 300/75 mg/m 2 ) twice daily taken with food, up to a maximum dose of 533/133 mg in children >45 kg twice daily should be considered when used in combination with efavirenz or nevirapine in treatment experienced children 6 months to 12 years of age in which reduced susceptibility to lopinavir in clinically suspected (by treatment history or laboratory evidence). The following table contains dosing guidelines for KALETRA oral solution based on body weight, when used in combination with efavirenz or nevirapine in children (see CLINICAL PHARMACOLOGY --Drug Interactions and/or PRECAUTIONS --Table 6).
|
||||||||||||||||||||||||||||||||||||||||||
KALETRA (lopinavir/ritonavir) capsules are orange soft gelatin capsules imprinted with the corporate logo
![]() and the Abbo-Code PK. KALETRA is available as 133.3 mg lopinavir/33.3 mg ritonavir capsules in the following package sizes:
and the Abbo-Code PK. KALETRA is available as 133.3 mg lopinavir/33.3 mg ritonavir capsules in the following package sizes:
Bottles of 180 capsules each .................... ( NDC 0074-3959-77)
Recommended storage: Store KALETRA soft gelatin capsules at 36°F-46°F (2°C-8°C) until dispensed. Avoid exposure to excessive heat. For patient use, refrigerated KALETRA capsules remain stable until the expiration date printed on the label. If stored at room temperature up to 77°F (25°C), capsules should be used within 2 months.
KALETRA (lopinavir/ritonavir) oral solution is a light yellow to orange colored liquid supplied in amber-colored multiple-dose bottles containing 400 mg lopinavir/100 mg ritonavir per 5 mL (80 mg lopinavir/20 mg ritonavir per mL) packaged with a marked dosing cup in the following size:
160 mL bottle .......................................... ( NDC 0074-3956-46)
Recommended storage: Store KALETRA oral solution at 36°F-46°F (2°C-8°C) until dispensed. Avoid exposure to excessive heat. For patient use, refrigerated KALETRA oral solution remains stable until the expiration date printed on the label. If stored at room temperature up to 77°F (25°C), oral solution should be used within 2 months.
Ref: 03-5113-R4
Revised: March, 2001
ABBOTT LABORATORIES
NORTH CHICAGO, IL 60064, U.S.A.
 |
 |
KALETRA™
(lopinavir/ritonavir) capsules
(lopinavir/ritonavir) oral solution
ALERT: Find out about medicines that should NOT be taken with KALETRA. Please also read the section "MEDICINES YOU SHOULD NOT TAKE WITH KALETRA."
KALETRA™
(kuh-LEE-tra)
Generic Name: lopinavir/ritonavir
(lop-IN-uh-veer/rit-ON-uh-veer)
Read this leaflet carefully before you start taking KALETRA. Also, read it each time you get your KALETRA prescription refilled, in case something has changed. This information does not take the place of talking with your doctor when you start this medicine and at check ups. Ask your doctor if you have any questions about KALETRA.
KALETRA is a combination of two medicines. They are lopinavir and ritonavir. KALETRA is a type of medicine called an HIV (human immunodeficiency virus) protease (PRO-tee-ase) inhibitor. KALETRA is always used in combination with other anti-HIV medicines to treat people with human immunodeficiency virus (HIV) infection. KALETRA is for adults and for children age 6 months and older.
HIV infection destroys CD4 (T) cells, which are important to the immune system. After a large number of T cells are destroyed, acquired immune deficiency syndrome (AIDS) develops.
KALETRA blocks HIV protease, a chemical which is needed for HIV to multiply. KALETRA reduces the amount of HIV in your blood and increases the number of T cells. Reducing the amount of HIV in the blood reduces the chance of death or infections that happen when your immune system is weak (opportunistic infections).
KALETRA does not cure HIV infection or AIDS. The long-term effects of KALETRA are not known at this time. People taking KALETRA may still get opportunistic infections or other conditions that happen with HIV infection. Some of these conditions are pneumonia, herpes virus infections, and Mycobacterium avium complex (MAC) infections.
KALETRA does not reduce the risk of passing HIV to others through sexual contact or blood contamination. Continue to practice safe sex and do not use or share dirty needles.
It is important that you do not miss any doses. If you miss a dose of KALETRA, take it as soon as possible and then take your next scheduled dose at its regular time. If it is almost time for your next dose, do not take the missed dose. Wait and take the next dose at the regular time. Do not double the next dose.
If you suspect that you took more than the prescribed dose of this medicine, contact your local poison control center or emergency room immediately.
As with all prescription medicines, KALETRA should be kept out of the reach of young children. KALETRA liquid contains a large amount of alcohol. If a toddler or young child accidentally drinks more than the recommended dose of KALETRA, it could make him/her sick from too much alcohol. Contact your local poison control center or emergency room immediately if this happens.
Together with your doctor, you need to decide whether KALETRA is right for you.
KALETRA may interact with other medicines, including those you take without a prescription. You must tell your doctor about all the medicines you are taking or planning to take before you take KALETRA.
MEDICINES YOU SHOULD NOT TAKE WITH KALETRA.
It is possible that your doctor may need to increase or decrease the dose of other medicines when you are also taking KALETRA. Remember to tell your doctor all medicines you are taking or plan to take.
Before you take Viagra® (sildenafil) with KALETRA, talk to your doctor about problems these two medicines can cause when taken together. You may get increased side effects of VIAGRA, such as low blood pressure, vision changes, and penis erection lasting more than 4 hours. If an erection lasts longer than 4 hours, get medical help right away to avoid permanent damage to your penis. Your doctor can explain these symptoms to you.
Talk to your doctor or other health care provider if you have any questions about this medicine or your condition. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information Leaflet. If you have any concerns about this medicine, ask your doctor. Your doctor or pharmacist can give you information about this medicine that was written for health care professionals. Do not use this medicine for a condition for which it was not prescribed. Do not share this medicine with other people.
* The brands listed are trademarks of their respective owners and are not trademarks of Abbott Laboratories. The makers of these brands are not affiliated with and do not endorse Abbott Laboratories or its products.
Ref: 03-5113-R4
Revised: March, 2001
ABBOTT LABORATORIES
NORTH CHICAGO, IL 60064, U.S.A.